CAR NK-cells project, February 2025 update
A project update from Dr Ciprian Tomuleasa.
We successfully designed and tested in vitro CAR T and CAR NK cells, transfected with the iCas9 suicide trigger.
Thanks to the support of the EHA SWG ‘seeding call’ grant received in 2023, we were able to:
- Design a CAR T and CAR NK-based therapy for multiple myeloma and B-cell lymphomas
- Test the therapy in the preclinical setting
Challenges
CAR-based cancer immunotherapy was listed as one of the breakthroughs in modern hematology and oncology. The development of CAR T-cell therapy for select hematological malignancies represents one of the most remarkable cancer therapeutic advances in the past decade.
Currently, CD19-targeted CAR T-cell therapy is approved for relapsed/refractory diffuse large B-cell lymphoma and acute lymphoblastic leukemia. Despite the successes achieved to date, there remain significant challenges associated with CAR T-cell therapy and substantial research efforts are underway to develop new targets and approaches.
Despite their initial success, CAR-T based therapies face a series of limitations. Specifically, their limited array of targets restricts their therapeutic utility to only a limited number of hematological malignancies. Treatment-related adverse effects, such as cytokine release syndrome (CRS), are a common occurrence.
The side effects of CAR T cells result from an aberrantly upregulation of CAR T cell activity. Therefore, it is crucial to control the CAR T cell activity whenever the patient is at risk.
The iCas9 system induces apoptosis in CAR T cells through caspase-9 dimerization by compound. This is a potentially valid alternative to controlling the activity, and also the persistence and survival, of the CAR T cells following infusion.
‘Suicide switches’ have previously been reported to be valid alternatives for on/off switches that limit the side effects of CAR T cells—one alternative being dasatinib. However, a ‘built-in’ switch might potentialy be more efficient. This is the case of the small molecule-controlled, inducible caspase-9 (iCas9) systems, which can afford the ability to selectively eliminate engineered T cells (Figure 1).
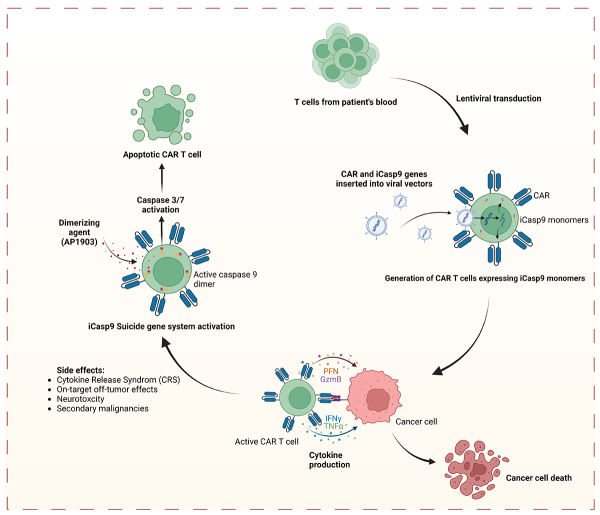
This can potentially be a valid alternative and eliminate the unwanted side-effects of uncontrollable CAR T cells following inclusion in the patient bloodstream. It could therefore potentially eliminate the risk of CRS, neurological side-effects, or even secondary malignancies.
Future plans
Our aim is to submit an abstract describing the major findings of this project at the next EHA Congress, to be held in Milan in June 2025, and possibly at the 2025 ASH meeting.