This information originally appeared on the previous EHA website. Links to other pages may be inactive.

This press release is originally found here: http://prn.to/2YF7Js3
THE HAGUE, Netherlands, Feb. 3, 2021 /PRNewswire/ -- T2EVOLVE is a new breakthrough alliance of academic and industry leaders in cancer immunotherapy under the European Union's Innovative Medicines Initiative (IMI). The key objective of T2EVOLVE is to accelerate development and increase awareness and access of cancer patients to immunotherapy with immune cells that harbor a genetically engineered T cell receptor (TCR) or synthetic chimeric antigen receptor (CAR). Simultaneously, T2EVOLVE aims to provide guidance on sustainable integration of these treatment into the EU healthcare system.
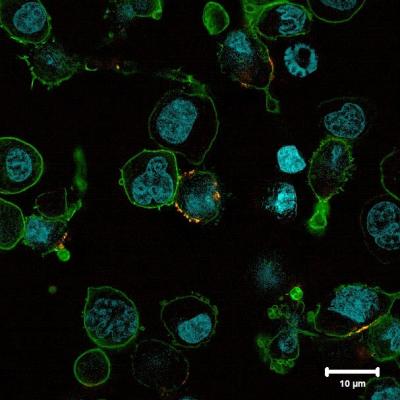
Figure 1: A tumor cell (left: cell nucleus [blue], cell surface [green]) is tackled by a reprogrammed immune cell (right: synthetic CAR [yellow]). ©Universitätsklinikum Würzburg. (PRNewsfoto/T2EVOLVE)
The clinical development of engineered T-cell treatments is moving ahead and the EU has already approved this therapy for the treatment of leukemia. Despite its potential to cure many types of cancers and other infectious or autoimmune diseases, the translation into clinical trials and entry into the EU market is slow and lagging behind other regions. There is a lack of (1) standardized safety and efficacy prediction models, (2) strategies for optimal patient conditioning, and (3) customized manufacturing, release, and monitoring schemes.
Goals of the T2EVOLVE consortium
- Optimize pre-clinical models for safety and efficacy prediction
- Define gold standard patient monitoring methods
- Select optimal lymphodepletion regimens to improve therapy reception
- Produce Good Medical Practice guidance and establish standard product profiles
- Integrate patient stakeholders and improve patient experience
- Expand patient access
T2EVOLVE members
T2EVOLVE includes innovators and pioneers in the field of immunotherapy from 9 nations and is coordinated by the Universitätsklinikum Würzburg, Germany, and Servier, France. Visit this page to view the full list of partners.
As a member, the European Hematology Association (EHA) supports the development of advanced, safe, effective, and accessible engineered T-cell therapies. EHA will be responsible for the dissemination of information to assist the implementation of materials dedicated to educating patients and their families as well as a wide range of resources for Health Care Professionals. Finally, EHA will be part of a technical committee working towards analytical standards and endorsements of scientific methods.
Further information on the project will be available on the website www.t2evolve.eu and on the project's LinkedIn profile 'T2EVOLVE consortium'.
The T2EVOLVE project will have its first public appearance at the EHA-EBMT 3rd European CAR-T Cell Meeting (Virtual, February 4-6, 2021) with a workshop on February 5 at 7:00PM CET entitled 'How to rapidly move new CAR-Ts forward from bench to bedside? Key questions and answers!'
This project receives funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 945393. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation program and EFPIA.
To read the full press release, visit: https://t2evolve.eu/latest-news