Regulation on Health Technology Assessment
The Regulation on Health Technology Assessment (HTAR) was proposed by the European Commission in 2018. It was formally adopted in December 2021 and will apply from January 2025.
Scope of the HTAR
The legislation covers the clinical aspects of Health Technology Assessment (HTA). This means the relative clinical effectiveness of a new health technology, when compared with medicines or therapies already approved for the European market.
Under the HTAR, safety and effectiveness will be assessed via Joint Clinical Assessments (JCAs), conducted by the Member States’ HTA bodies.
In addition, the HTAR also provides for:
- Joint Scientific Consultations, to advise technology developers on clinical study designs that generate appropriate evidence
- Horizon-scanning exercises, to identify promising health technologies at an early stage and help health systems prepare for them
Not covered by the Regulation
The following responsibilities remain the exclusive domain of the individual Member States:
- Economic evaluation, i.e. cost-benefit assessment
- Pricing and reimbursement decisions
- Ethical and legal considerations
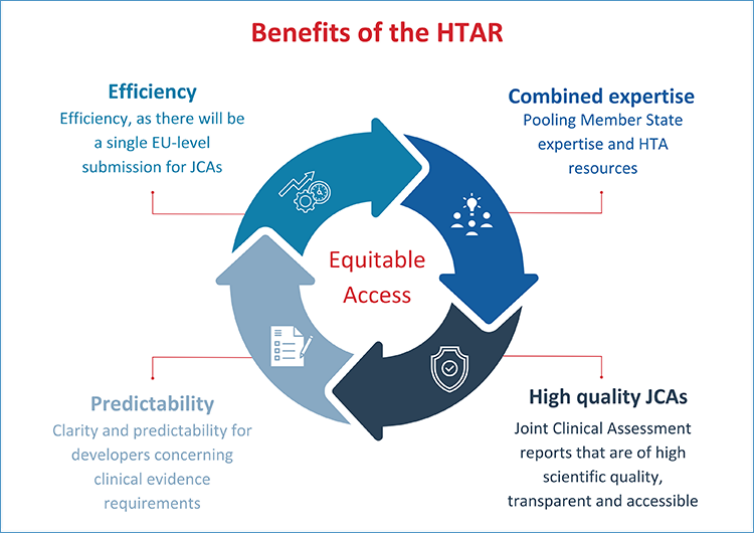
Foreseen benefits
- Pooling member state expertise and HTA resources.
- Joint Clinical Assessment reports that are of high scientific quality, transparent and accessible.
- Clarity and predictability for developers concerning clinical evidence requirements.
- Efficiency, as there will be a single EU-level submission for JCAs.
As a result, the HTAR will be crucial for improving timely and equitable access to innovative therapies across the EU.
Stepwise application
- January 12, 2025: new oncology medicines and advanced therapy medicinal products.
- January 13, 2028: orphan medicinal products.
- January 13, 2030: all new medicines will come under the scope of the Regulation.
EHA involvement
EHA has been heavily involved in shaping the HTAR, first during the legislative process, when EHA co-lead the BioMed Alliance’s positioning on joint HTA.
EHA was subsequently selected as a member of the formal HTA Stakeholder Network, launched in June 2023 to facilitate the involvement of stakeholders in the implementation of the Regulation.
Focus of EHA's advocacy
EHA's advocacy on EU HTA has focused on the following areas.
Involvement of clinical experts
In our advocacy, we have emphasized that the involvement of clinical experts should be:
- Timely
- Structural
- Meaningful
Structural and effective involvement of medical societies
We have highlighted the importance of the structural and effective involvement of medical societies, especially on the following issues:
- Expert identification and selection
- The educating and training of clinicians on HTA
- Advice on methodologies and guidance documents
- Establishing standards of care (clinical practice guidelines)
- Aligning with patient representatives and supporting their involvement
Overcoming practical challenges
In our advocacy, we have signaled challenges that may affect the effective involvement of clinical experts and medical societies. By extension, these issues could affect the quality and impact of EU HTA.
Examples of these challenges include:
- Timelines
- Conflict of Interest policies
- The facilitation and recognition of the value of clinicians' contribution to HTA by employers (such as hospitals or universities)
Harmonization
We have emphasized that a successful HTAR requires real harmonization in terms of:
- The formulation of PICO criteria (by national HTA bodies)
- The alignment of evidence generation (EMA-HTA-payers)
- Expert selection (not only at the national but at the European level)



 Back
Back